 Multiple Choice Questions
Multiple Choice QuestionsThe vapour pressure of acetone at 20oC is 185 torr. When 1.2 g of a non-volatile substance was dissolved in 100 g of acetone at 20oC, its vapour pressure was 183 torr. The molar mass (g mol-1 ) of the substance is:
32
64
128
128
B.
64
Given,
po = 185 torr at 20oC
ps = 183 torr at 20oC
Mass of non-volatile substance,
m= 1.2 g
Mass of acetone taken = 100 g
As we have,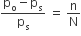
putting the values, we get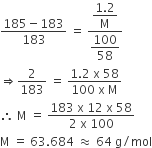
Two Faraday of electricity is passed through a solution of CuSO4. The mass of copper deposited at the cathode is: (at. mass of Cu = 63.5 amu)
0 g
63.5 g
2 g
2 g
Higher order (>3) reactions are rare due to:
the increase in entropy and activation energy as more molecules are involved.
shifting of equilibrium towards reactants due to elastic collisions
loss of active species on a collision
loss of active species on a collision
3 g of activated charcoal was added to 50 mL of acetic acid solution (0.06N) in a flask. After an hour it was filtered and the strength of the filtrate was found to be 0.042 N. The amount of acetic acid adsorbed (per gram of charcoal) is:
18 mg
36 mg
42 mg
42 mg
In the context of the Hall-Heroult process for the extraction of Al, which of the following statements is false?
CO and CO2 are produced in this process
Al2O3 is mixed with CaF2 which lowers the melting point of the mixture and brings conductivity
Al3+ is reduced at the cathode to form Al
Al3+ is reduced at the cathode to form Al
Match the catalysis to the correct processes.
| Catalyst | Process |
| (A)TiCl3 | (i) Wacker Process |
| (B) PdCl2 | (ii) Ziegler -Natta |
| (C) CuCl2 | (iii) Contact process |
| (D) V2O5 | (iv)Deacon's process |
(A)- (iii), (B)-(ii), (C)-iv, (D)- (i)
(A) - (ii), (B) - (i), (C) -(iv), (D) - (iii)
(A)- (ii), (B)- (iii), (C)- (iv), (D) - (i)
(A)- (ii), (B)- (iii), (C)- (iv), (D) - (i)
The number of geometric isomers that can exist for square planar [Pt (Cl) (py) (NH3) (NH2OH)]+ is (py = pyridine)
2
3
4
4
The Colour of KMnO4 is due to
M → L charge transfer transition
d-d transition
L →M charge transfer transition
L →M charge transfer transition
