 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following will most readily be dehydrated in acidic solution?
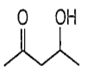
![]()
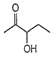
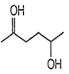
A.

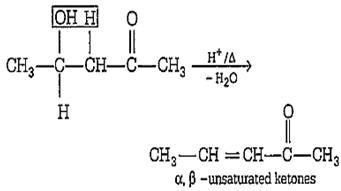
Among the given compounds, the compound present in option (a) is readily dehydrated in acidic medium due to presence of acidic hydrogen with -OH group.
In option (d), the strength of acidic hydrogen is less.
Which is the correct statement?
Hydrogen peroxide can oxidise permanganate ion.
Permanganate ion can oxidise manganous ion.
Manganate ion is more stable than permanganate ion.
Permanganate ion cannot be reduced to manganate ion
Vapour pressure of a solution at 100° C having 3.42 g of cane sugar in 180 g water is
759.2 mm
760 mm
740 mm
748.5 mm
The correct representation of a complex ion is
[Co(H2O)(NH3)4Cl]2+
[CoCl(H2O)(NH3)4]2+
[Co(NH3)4Cl(H2O)]2+
[Co(NH3)4(H2O)Cl]2+
Calcination is used in metallurgy for removal of
water and sulphide
water and CO2
CO2 and H2S
H2O and H2S
75% of a first order reaction was completed in 32 min. When was 50% of the reaction completed?
In 16 min
In 24 min
In 8 min
In 4 min
The activation energy of a reaction is zero. The rate constant of this reaction
Increases with an increase of temperature
decreases with an increase of temperature
decreases with decrease of temperature
is independent of temperature
