 Multiple Choice Questions
Multiple Choice QuestionsA metal crystallises in a face centred cubic structure. If the edge length of its unit cell is'a', the closest approach between two atoms in metallic crystal will be
2a



D.

In FCC unit cell atoms are in constant along face diagonal
So, √2a = 4R
therefore, the closest distance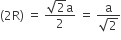
Two reactions R1 and R2 have identical pre-exponential factors. Activation energy of R1 exceeds that of R2 by 10 kJ mol–1. If k1 and k2 are rate constants for reactions R1 and R2 respectively at 300 K, then ln(k2/k1) is equal to-
(R = 8.314 J mol–1K–1)
8
12
6
6
The Tyndall effect is observed only when following conditions are satisfied:-
(a) The diameter of the dispersed particles is much smaller than the wavelength of the light used.
(b) The diameter of the dispersed particle is not much smaller than the wavelength of the light used.
(c) The refractive indices of the dispersed phase and dispersion medium are almost similar in magnitude.
(d) The refractive indices of the dispersed phase and dispersion medium differ greatly in magnitude.
(a) and (d)
(b) and (d)
(a) and (c)
(a) and (c)
Which of the following reactions is an example of a redox reaction?
XeF4 + O2F2 → XeF6 + O2
XeF2 + PF5 → [XeF]+PF6–
XeF6 + H2O → XeOF4 + 2HF
XeF6 + H2O → XeOF4 + 2HF
The products obtained when chlorine gas reacts with cold and dilute aqueous NaOH are
ClO– and ClO3–
ClO2- and ClO3-
Cl– and ClO–
Cl– and ClO–
Sodium salt of an organic acid 'X' produces effervescence with conc. H2SO4. 'X' reacts with the acidified aqueous CaCl2 solution to give a white precipitate which decolourises acidic solution of KMnO4. 'X' is
C6H5COONa
HCOONa
Na2C2O4
Na2C2O4
The freezing point of benzene decreases by 0.45°C when 0.2 g of acetic acid is added to 20 g of benzene. If acetic acid associates to form a dimer in benzene, percentage association of acetic acid in benzene will be
(Kf for benzene = 5.12 K kg mol–1)
64.6%
80.4%
74.6%
74.6%
On treatment of 100 mL of 0.1 M solution of CoCl3 . 6H2O with excess AgNO3; 1.2 × 1022 ions are precipitated. The complex is
[Co(H2O)4 Cl2]Cl.2H2O
[Co(H2O)3Cl3].3H2O
[Co(H2O)6]Cl3
[Co(H2O)6]Cl3
1 gram of a carbonate (M2CO3) on treatment with excess HCl produces 0.01186 mole of CO2. The molar mass of M2CO3 in g mol-1 is
1186
84.3
118.6
118.6
