 Multiple Choice Questions
Multiple Choice QuestionsThe crystal field stabilization energy (CFSE) of [Fe(H2O)6]Cl2 and K2[NiCl4], respectively are:
-2.4 Δ0 and -1.2Δt
-0.4 Δ0 and -1.2Δt
-0.6 Δ0 and -0.8Δt
-0.4 Δ0 and -0.8Δt
D.
-0.4 Δ0 and -0.8Δt
[Fe(H2O)6]Cl2: d6t2g4eg2: [(-0.4) 4 + 2(0.6)]Δ0
K2[NiCl4] d8eg4t2g4:[(0.6)4+(0.4)4]Δt= -0.8Δt
1 g of a non-volatile non – electrolyte solute is dissolved in 100 g of two different solvents A and B whose ebulliscopic constants are in the ratio of 1:5. The ratio of the elevation in their boiling points, is:
1:0.2
10: 1
5: 1
1: 5
The correct order of the first ionization enthalpies is :
Ti < Mn < Ni < Zn
Mn < Ti < Zn < Ni
Ti < Mn < Zn < Ni
Zn < Ni < Mn < Ti
The number of pentagons in C60 and trigons (triangles ) in white phosphours, respectively ,are :
20 and 4
20 and 3
12 and 4
12 and 3
For the reaction of H2 with I2, the rate constant is 2.5 × 10-4 dm3 mol-1s-1 at 327°C and 1.0 dm3 mol-1s-1 at 527°C. The activation energy for the reaction, in kJ mol-1 is: (R= 8.314 Jk-1mol-1)
166
150
59
72
The correct statement is :
Zincite is a carbonate ore.
Zone refining process is used for the refining of titanium
Aniline is a for the stabilizer
Sodium cyanide cannot be used in the metallurgy of silver
The highest possible oxidation states of uranium plutonium , respectively are
6 and 7
7 and 6
4 and 6
6 and 4
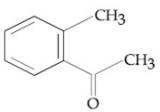
Compound A(C9H10O) shows positive iodoform rest. Oxidation of A with KMnO4/ KOH gives B(C8H6O4). Anhydride of B is used for the preparation of phenolpthalein. Compound A is:
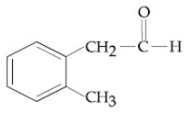
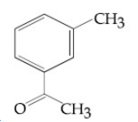
![]()
Which of the following is NOT a correct method if the preparation of benzylamine from cyanobenzene ?
H2/ Ni
(i) LiAlH4
(ii) H3O+
(i) SnCl2 + HCl(gas)
(ii) NaBH4
(i) HCl/H2O
(ii) NaBH4
