 Multiple Choice Questions
Multiple Choice QuestionsIf Z is the number of atoms in the unit cell that represents the closest packing sequence... A B C A B C..., the number of tetrahedral voids in the unit cell is equal to
Z
2Z
Z/2
Z/4
B.
2Z
In the above given question, the total number of tetrahedral voids in the unit cell is equal to 2Z.
Number of tetrahedral voids = 2 × number of sign = 2Z
For reaction aA → xP, when [A] = 2.2 mM, the rate was found to be 2.4 mM s-1. On reducing concentration of A to half, the rate changes to 0.6 mM s-1. The order of reaction with respect to A is
1.5
2.0
2.5
3.0
Assertion : Reaction of SO2 and H2S in the presence of Fe2O3 catalyst gives elemental sulphur.
Reason : SO2 is a reducing agent.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : SiF62- is known but SiCl62- is not.
Reason : Size of fluorine is small and its lone pair of electrons interacts with d-orbitals of Si strongly.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Addition of NH4OH to an aqueous solution of BaCl2 in the presence of NH4Cl (excess) precipitates Ba(OH)2.
Reason : Ba(OH)2 is insoluble in water.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : The molecular weight of acetic acid determined by depression in freezing point method in benzene and water was found to be different.
Reason : Water is polar and benzene is non-polar.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Galvanised iron does not rust.
Reason : Zinc has a more negative electrode potential than iron.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Extraction of iron metal from iron oxide ore is carried out by heating with coke.
Reason : The reaction Fe2O3 (s) → Fe (s) + O2 (g) is a spontaneous process.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
The major product formed in the following reaction is
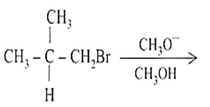
(CH3)2CH-CH2OCH3
CH3-CH(OCH3)-CH2CH3
CH3-C(CH3)=CH2
(CH3)2-C-OCH3- CH3
The major product obtained on treatment of CH3CH2CH(F)CH3 with CH3O/CH3OH is
CH3CH2CH(OCH3)CH3
CH3CH = CHCH3
CH3CH2CH = CH2
CH3CH2CH2CH2OCH3
