 Multiple Choice Questions
Multiple Choice QuestionsAssertion : Al forms [AlF6]3- but B does not form [BF6]3-.
Reason : B does not react with fluorine.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
C.
If assertion is true but reason is false
Al forms [AlF6]3- but B does not form [BF6]3- because B does not have any vacant d-orbital or sub-shell as its valence shell.
Assertion : Cu(OH)2 is soluble in NH4OH but not in NaOH.
Reason : Cu(OH)2 forms a soluble complex with NH3.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
2-Phenylethylbromide when heated with NaOEt, elimination takes place. No deuterium exchange takes place when the reaction is carried out in C2H5OD solvent. The mechanism will be
E1 elimination
E2 elimination
E1 cB elimination
E2 or E1cB
Tincture of iodine is
aqueous solution of I2
solution of I2 in aqueous KI
alcoholic solution of I2
aqueous solution of KI
Decreasing order of stability of ions is.
(i) CH3 - H - CH3
(ii) CH3 - H - OCH3
(iii) CH3 - H - COCH3
(i) > (ii) > (iii)
(ii) > (i) > (iii)
(ii) > (iii) > (i)
(iii) > (i) > (ii)
Consider the reaction,
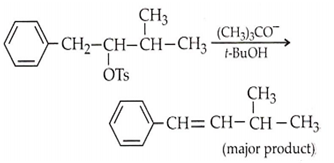
The correct explanation is
the product is formed due to nucleophilic substitution
the product is formed according to Saytzeff's rule
conjugated double bond product is formed, due to higher stability because of resonance stabilization
(CH3)3CO- is a better leaving group
In solvents like DMSO, acetonitrile, F- ion of dissolved NaF is more reactive than in methyl alcohol becasue
CH3OH is more polar than DMSO and CH3CN
CH3OH is less polar than DMSO and CH3CN
unsolvated F- ion is DMSO or CH3CN acts more efficiently as nucleophile
-OH group is a better leaving group than F- ion
