 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following complex ions is not expected to absorb visible light?
[Ni(CN)4]2-
[Cr(NH3)6]3+
[Fe(H2O)6]2+
[Fe(H2O)6]2+
A.
[Ni(CN)4]2-
For the absorption of visible light, the presence of unpaired d- electrons is the necessity.
a) In [Ni(CN)4]2- , Ni is present as Ni2+
Ni2+ = [Ar] 3d8 4s0
Therefore, [Ni(CN)4]2- = 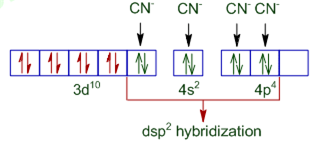
(pairing occurs because CN- is a strong field ligand).
Since in [NiCN)4]2-, no unpaired electron is present in d- orbitals, it does not absorb visible light.
(b) In [Cr(NH3)6]3+, [Fe(H2O)6]2+ [Ni(H2O)6]2+ has unpaired electrons such as
[Cr(NH3)6]3+ Cr3+ = Have three unpaired electron
[Fe(H2O)6]2+ Fe2+ = Have four unpaired electrons
[Ni(H2O)6]2+ Ni2+ = Have two unpaired electrons
AB crystallises in a body centred cubic lattice with edge length 'a' equal to 387 pm. The distance between two oppositely charged ion in the lattice is
335 pm
250 pm
200 pm
200 pm
An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase?
Addition of NaCl
Addition of Na2SO4
Addition of 1.00 molal KI
Addition of 1.00 molal KI
For an endothermic reaction, energy of activation is Ea and enthalpy of reaction is ΔH (both of these in kJ/mol). Minimum value of Ea will be
less than ΔH
equal to ΔH
more than ΔH
more than ΔH
For the reaction of silver ions with copper metal, the standard cell potential was found to be +0.46 V at 25o C. The value of standard Gibbs energy, ΔGo will be (F = 96500 C mol-1 )
-89.0 kJ
-89.0 J
-44.5 kJ
-44.5 kJ
Which of the following ions will exhibit colour in aqueous solutions?
La3+ (Z = 57)
Ti3+ (Z = 22)
Lu3+
Lu3+
A solution of sucrose (molar mass = 342 g mol-1) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water. The freezing point of the solution obtained will be (kf for water = 1.86 K kg mol-1)
-0.372o C
-0.520o C
+0.372o C
+0.372o C
An increase in equivalent conductance of strong electrolyte with dilution is mainly due to
the increase in ionic mobility of ions
100% ionisation of electrolyte at normal dilution
the increase in both, ie, the number of ions and ionic mobility of ions
the increase in both, ie, the number of ions and ionic mobility of ions
Crystal field stabilisation energy for high spin d4 octahedral complex is
-1.8 Δo
-1.6 Δo + P
-1.2 Δo
-1.2 Δo
