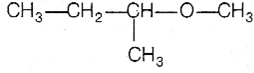Multiple Choice Questions
Multiple Choice QuestionsAn excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaquachromium (III) chloride. The number of moles of AgCl precipitate would be
0.001
0.002
0.003
0.003
A.
0.001
The formula of dichlorotetraqua chromium (III) chloride is [Cr(H2O)Cl2]Cl.
On ionisation it generates only one Cl- ion.
[Cr(H2O)4Cl2]Cl --> [Cr(H2O)Cl2]+ +Cl-
Initial 100x0.01 0 0
KMnO4 can be prepared from K2MnO4 as per reaction
The reaction can go to completion by removing OH- ions by adding
HCl
KOH
CO2
CO2
Which of the following statements about the interstitial compounds is incorrect?
They retain metallic conductivity
They are chemically reactive
They are much harder than the pure metal
They are much harder than the pure metal
The reaction by which benzaldehyde cannot be prepared?
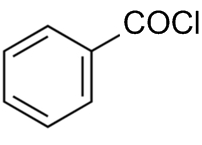 +H2 in presence of anhydrous AlCl3.
+H2 in presence of anhydrous AlCl3.
Which of these is not a monomer for high molecular mass silicone polymer?
MeSiCl3
Me2SiCl2
Me3SiCl
Me3SiCl
Antiseptics and disinfectants either kill or prevent the growth of microorganisms. Identify which of the following is not true.
A 0.2% solution of phenol is an antiseptic while 1% solution acts as a disinfectant
Chlorine and iodine are used as strong disinfectants
Dilute solutions of boric acid and hydrogen, peroxide are strong antiseptics
Dilute solutions of boric acid and hydrogen, peroxide are strong antiseptics
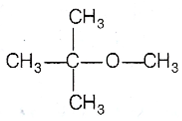
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI?