 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following is not a common component of photochemical smog?
Ozone
Acrolein
Peroxyacetyl nitrate
Peroxyacetyl nitrate
A.
Ozone
Among the given chlorofluorocarbons are the compounds that are responsible for ozone depletion which degrades ozone into molecular oxygen. It is not a component of photochemical smog. while other given compounds are the main components of photochemical smog.
If a is the length of the side of a cube, the distance between the body centred atom and one corner atom in the cube will be
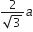
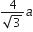
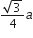
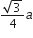
Which property of colloids is not dependent on the charge on colloidal particles?
Coagulation
Electrophoresis
Electro-osmosis
Electro-osmosis
Of the following 0.10 m aqueous solutions, which one will exhibit the largest freezing point depression?
KCl
C6H12O6
Al2(SO4)3
Al2(SO4)3
When 0.1 mol MnO42- is oxidised, the quantity of electricity required to completely oxidise MnO42- to MnO42- is
95600C
2 x 96500C
9650 C
9650 C
Using the Gibbs energy change, ΔG0 = +63.3 KJ for the following reaction,
Ag2CO3 (s) r2Ag+ (aq) + CO32- (aq)
the Ksp of Ag2CO3 (s) in water at 250 C is (R= 8.314 JK-1 mol-1)
3.2 x 10-26
8.0 x 10-12
2.9 x 10-3
2.9 x 10-3
The weight of silver (at. wt. = 108) displaced by a quantity of electricity which displaces 5600 mL of O2 at STP will be
5.4 g
10.8 g
54.0 g
54.0 g
Acidity of diprotic acids in aqueous solutions increases in the order
H2S < H2Se< H2Te
H2Se <H2S <H2Te
H2Te <H2S < H2Se
H2Te <H2S < H2Se
In acidic medium,H2O2 changes Cr2O72- to CrO5 which has two (-O-O-) bonds. Oxidation state of Cr in CrO3 is
+5
+3
+6
+6
Among the following complexes, the one which shows zero crystal field stabilisation energy (CFSE) is
[Mn(H2O)6]3+
[Fe(H2O)6]3+
[Co(H2O)6]3+
[Co(H2O)6]3+
