 Multiple Choice Questions
Multiple Choice QuestionsFor an ideal binary liquid solution with in which relation between Xx (mole fraction of X in liquid phase) and Yx ( mole fraction of Y in liquid and vapour phase respectively.
Xx > YX
Xx = Yx
XX, YX, XY and YY cannot be correlated
C.
Point out the incorrect reaction from the following.
2Na2CrO4 + H+ Na2Cr2O7 + 2Na+ + H2O
4MnO2 + 4KOH + O2 4KMnO4 + 2H2O
2Mn + 5C2 + 16H+ 2Mn2+ + 10CO2 + 8H2O
Mn + 8H+ + 5Fe2+ 5Fe3+ + Mn2+ + 4H2O
The conductivity of 0.001028 mol L-1 acetic acid is 4.95 x 10-5 S cm-1. Find out its dissociation constant if Λm for acetic acid is 390.5 S cm-1 mol-1.
2.18 x 10-5 mol-1L-1
1.78 x 10-5 mol L-1
3.72 x 10-4 mol L-1
2.37 x 10-4 mol L-1
A hypothetical reaction
X2 + Y2 2XY follows the following mechanism
X2 X + X .... fast
X + Y2 XY + Y .... slow
X + Y XY .... fast
The order of overall reaction is
2
1
0
The major role of fluorspar (CaF2) which is added in small quantity in the electrolytic reduction of alumina dissolved in fused cryolite (Na3AlF6) is
I. as a catalyst.
II. to make the fused mixture very conducting.
III. to lower the temperature of melting.
IV. to decrease the rate of oxidation of carbon at the anode.
I, II
II, III
I, II, III
III, IV
The variation of concentration of the product P with time in the reaction, A P is shown in following graph.
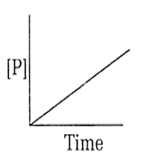
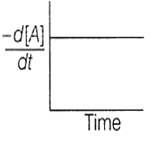
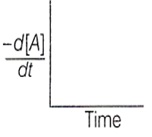
The graph between and time will be of the type


In an experiment, addition of 4.0 mL of 0.005 M BaCl2 to 16.0 mL of arsenious sulphide sol just causes complete coagulation in 2 h. The flocculating value of the effective ion is
Ba2+, 1.0
Ba2+, 2.0
Cl-, 1.0
Cl-, 2.0
The aqueous solution of an unknown sodium salt gives the following reactions.
I. It decolourises a solution of iodine in potassium iodide.
II. It gives white turbidity with dil.HCl solution.
III. It gives a white precipitate with AgNO3 solution which changes colours and finally becomes black on standing.
The unknown sodium salt is
Sodium thiosulphate
sodium bisulphite
sodium sulphite
sodium sulphide
