 Multiple Choice Questions
Multiple Choice Questions![]()
Identify the end product:
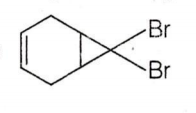
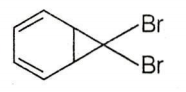
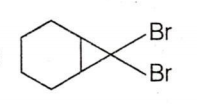
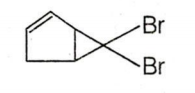
C.

CBr4 + MeLi MeBr + LiCBr3
LiCBr3 LiBr + CBr3
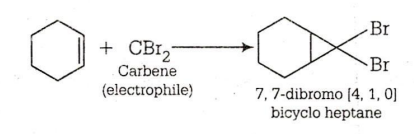
In the given reaction ,
R-OH + HX RX + H2O
the order of reactivity of alcohols is:
tertiary < secondary < primary
tertiary > secondary > primary
tertiary < secondary > primary
secondary > primary > tertiary
In the given reaction,
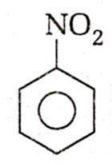 X Y
X Y
The products X and Y respectively are:
o-bromo nitrobenzene and o-bromoaniline
p-bromo nitrobenzene and p-bromoaniline
m-bromo nitrobenzene and m-bromoaniline
m-bromo nitrobenzene and m-bromoaniline
Which one of the following reactions cannot be used for the reduction of:
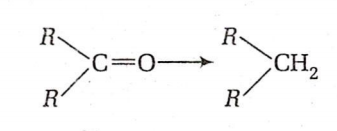
HI and red phosphorus at 200C
Wolff-Kishner reduction
Clemmensen reduction
Wurtz reaction
Buna-N synthetic rubber is a copolymer of:
H2C=CH-CN and H2C=CH-CH=CH2
H2C=CH-CN and H2C=CH-=CH2
H2C=CH-=CH and H2C=CH-CH=CH2
H2C=CH-CH=CH2 and H5C6-CH=CH2
What is the role of bithional in toilet soaps?
it controls the pH of the skin and do not harm it
it reduces the odours produced by bacterial decomposition of organic matter
it helps in improving the colour of the skin
it act like an antihistamine
The true statement regarding sucralose is:
trichloro derivative of sucrose
it does not provide calories
it is stable at cooking temperature
All of the above
A compound is soluble in conc. H2SO4 . It does not decolourise bromine in CCL4 but in oxidised by chromic anhydride in aqueous H2SO4 with 2s , turning orange solution to blue green , afterward opaque. The original solution contains :
a primary alcohol
a secondary alcohol
a tertiary alcohol
an ether
