 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following aqueous solution should have a highest boiling point?
1.0 M NaOH
1.0 M Na2SO4
1.0 M NH4NO3
10 MKNO3
B.
1.0 M Na2SO4
It depends on the Van't Hoff factor.
Van't Hoff factor is directly proportional to the boiling point.
In the case of Na2SO3, i is maximum = 3
Hence it has maximum boiling points.
When a lead storage battery is discharged;
SO2 is evolved
lead sulphate is consumed
lead is formed
sulphuric acid is consumed
Combustion of glucose takes place according to the equation.
C6H12O6 + 6O2 → 6CO2 + 6H2O
Δ = - 72 k-cal
The energy required for combustion of 1.6 g of glucose is
0.064 kcal
0.64 kCal
6.4 kcal
64 kcal
when the heat of reaction at constant pressure is -2.5 x 103 cal can entropy change is 7.4 cal deg-1 at 25oC, the reaction is predicted as
reversible
Spontaneous
non -spontaneous
Irreversible
For the chemical reaction,
2O3 ⇌ 3O2
The reaction proceeds as follows
O3 ⇌ O2 + O
O + O3 → 2O2 (slow)
The rate law expression will be
r = k' [O3]2
r = k' [O3]2[O2]-1
r = k'[O3][O2]
unpredictable
Among the following compounds, which will produce POCl3 with PCl5
Only O2
O2 and CO2
CO2, O2 and P4O10
SO2, H2O, H2SO4 and P4O10
In the following graph
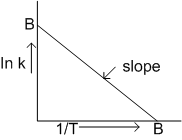
The slope of line AB give the information of the
value of
Value of
value of
value of
The value of reaction quotient [Q], for the following cell
Zn(s)|Zn2+ (0.01 M)|| Ag+ (1.25 M | Ag (s) is
156
125
1.25 x 10-2
6.4 x 10-3
In cyanide extraction process of silver from argentite ore, the oxidising and reducing agents are respectively.
O2 and CO2
O2 and Zn dust
HNO3 and Zn dust
HNO3 and CO
