 Short Answer Type
Short Answer TypeWrite the structure and IUPAC name of the major product obtained when ethyl magnesium bromide reacts with formaldehyde and the product is then hydrolysed with dilute H2SO4.
Compare the relative boiling points of:![]()
Phenol has a higher boiling point than thio phenol although thio phenol has higher molecular mass. This is due to the presence of intermolecular hydrogen bonding in phenol.
You are given benzene, conc. 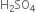 and NaOH. Write the equations for the preparation of phenol using these reagents.
and NaOH. Write the equations for the preparation of phenol using these reagents.
