Short Answer Type
Short Answer TypeWrite the structure and IUPAC name of the major product obtained when ethyl magnesium bromide reacts with formaldehyde and the product is then hydrolysed with dilute H2SO4.
You are given benzene, conc. 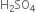 and NaOH. Write the equations for the preparation of phenol using these reagents.
and NaOH. Write the equations for the preparation of phenol using these reagents.
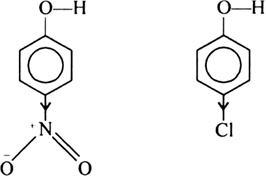
Both the chlorine and the nitro group at the para position makes the phenol group acidic because they are both electron withdrawing groups that stabilize the negative charge of phenol. However, the fact that the nitro groups is electron withdrawing goes resonance, allows better stabilize the negative charge of the oxygen then chlorine alone. Thus, p-nitrophenol is more acidic than p-chlorophenol.