 Short Answer Type
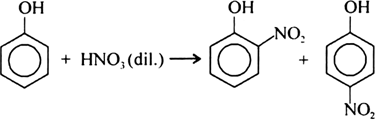
Short Answer TypeWhat happens when dilute HNO3 is added to phenol? Name the method to separate the isomers obtained.
With dilute nitric acid at low temperature (298 K),
phenol yields a mixture of ortho and para nitrophenols.
Give the structure of the products resulting from the reaction of sodium phenoxide with CO2 at 4 atm and 400 K followed by addition of aqueous acid.
While separating a mixture of ortho and para nitrophenols by stream distillation, name the isomer which is steam volatile. Give reason.
Name the reagent used in the conversion of phenol into 2, 4, 6-tribromophenol. Also write the equation.
