 Short Answer Type
Short Answer TypeWhat happens when dilute HNO3 is added to phenol? Name the method to separate the isomers obtained.
Give the structure of the products resulting from the reaction of sodium phenoxide with CO2 at 4 atm and 400 K followed by addition of aqueous acid.
p-nitrophenol is more soluble than ortho and para nitrophenol because P-nitrophenol has Inter-molecular Hydrogen Bonding while o-nitrophenol has Intra-molecular Hydrogen Bonding.
Now due to the Hydrogen Bonding more energy needs to be supplied to vaporize the compound as there are extra bonds other than that present bonds that hold the molecule.
There are bonds between molecules in p-nitrophenol which leads to higher boiling point as more energy need to be supplied to separate the molecules. Whereas in o-nitrophenol the bonds exist in the molecule itself due to which there is virtually no change in the boiling point.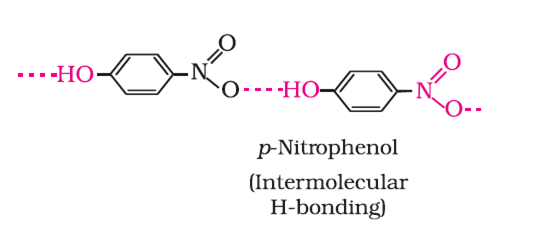
While separating a mixture of ortho and para nitrophenols by stream distillation, name the isomer which is steam volatile. Give reason.
Name the reagent used in the conversion of phenol into 2, 4, 6-tribromophenol. Also write the equation.
