Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeAn aromatic compound 'A' of molecular formula C7H7ON undergoes a series of reactions as shown below. Write the structures of A, B, C, D and E in the following reactions: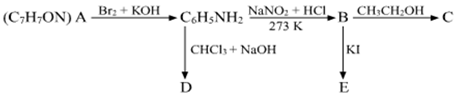
 Short Answer Type
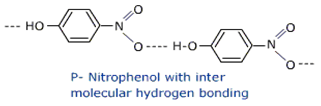
Short Answer TypeWhich of the following isomers is more volatile: o-nitrophenol or p-nitrophenol?
In o-nitro phenol, -OH is linked to –NO2 by means of intramolecular H-bonding so, it is highly volatile.
Explanation:
Stronger intermolecular forces would make the substance less volatile. So we can see intermolecular H-bonding in P-nitro phenol, so P-nitro phenol is less volatile, where as in o-nitro phenol intra H-bonding takes places that’s why it is highly volatile.