 Multiple Choice Questions
Multiple Choice QuestionsOut of the compounds below the vapour pressure of (B) at a particular temperature is
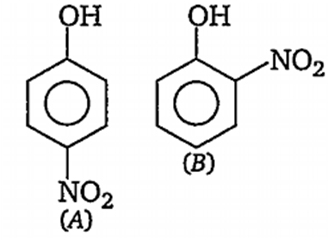
higher than that of (A)
lower than that of (A)
higher or lower than (A), depending on the size of the vessel
same as that of (A)
An oxygen containing organic compound upon oxidation forms a carboxylic acid as the only organic product with its molecular mass higher by 14 units. The organic compound is
an aldehyde
a primary alcohol
a secondary alcohol
a ketone
Phenol forms a tribromo derivative, 'X' is
bromine in benzene
bromine in water
potassium bromide solution
bromine in carbon tetrachloride at 0°C
The compound which gives turbidity immediately with Lucas reagent at room temperature is
butan-1-ol
butan-2-ol
2-methyl propan-2-ol
2-methyl propan-1-ol
The conversion of m-nitrophenol to resorcinol involves respectively
hydrolysis, diazotization and reduction
diazotization, reduction and hydrolysis
hydrolysis, reduction and diazotization
reduction, diazotization and hydrolysis
HCHO was treated with a reagent X. The product formed upon hydrolysis in the presence of an acid gave C2H5OH. The reagent X is
alcoholic KOH
alcoholic KCN
CH3MgI
aqueous KOH
Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence of dry ether?
diphenyl
propane
toulene
ethane
Power alcohol is a mixture of
80% petrol + 20% ethanol + small quantity of benzene
80% ethanol + 20% benzene + small quantity of petrol
50% Petrol + 50% ethanol + small quantity of benzene
80% petrol + 20% benzene + small quantity of ethanol
Which of the following is strongly acidic?
Phenol
o-cresol
p-nitrophenol
p-cresol
C.
p-nitrophenol
The acidity of phenols ts due to the greater resonance stabilization of the phenoxide ion relative to phenol. Therefore, electron withdrawing groups (EWG) like - NO2 which stabilize the phenoxide ion more by dispersing the negative charge will tend to increase the acidity of phenols.

On the other hand, electron donating groups (EDG) like-alkyl group destabilize the phenoxide ion by intensifying the negative charge relative to phenol tend to decrease the acidic strength of phenols.

As methyl group has + / effect and it is stronger at o-position than at p-position, (+ I effect decreases with distance) o-cresol is a weaker acid than p-cresol. Thus, the order of acidic strength.
p- nitrophenol > phenol > p- resol > o-cresol
