 Multiple Choice Questions
Multiple Choice QuestionsToluene on treatment with CrO3 and (CH3CO)2O followed by hydrolysis with dil HCl gives
benzaldehyde
benzoic acid
phenol
phenylacetaldehyde
Diacidic base is
CH2(OH)2
Ca(OH)2
CH3CH(OH)2
All of these
B.
Ca(OH)2
Bases are the substance which give -OH ions (hydroxyl ions) when dissolved in
water. If one molecule of a base gives two hydroxyl ions, it is called diacidic base.
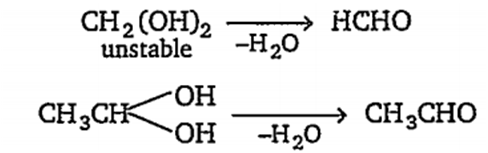
(i) CH2(OH)2 is a covalent compound and C-O bond is more stronger than O-H bond. Hence, it dissociates to give only a less amount of H+ ions instead of OH- ion and acts as acid.

(ii) Ca(OH)2 , being an ionic compound readily gives two hydroxyl ions when dissolved in water, hence it is a diacidic base.
Ca(OH)2 → Ca2+ + 2OH-
(iii) CH3CH(OH)2 is also a covalent compound and gives a small amount of H+ ions, hence it is an acid.

Actually when two-OH groups are attached with same carbon atoms, the compound is highly unstable and loses a water molecule, eg,
Phenol on treatment with diethyl sulphate in presence of NaOH give
phenetole
anisole
diphenyl ether
diethyl ether
The step which never be involved in the dehydration of alcohol is
protonation
elimination
hydride transfer
carbanion formation
The boiling point of ethanol is higher as compared to the boiling point of diethyl ether though both have the same molecular formula. This is due to
resonance
-R group
H-bonding
covalent bonding
Which one of the following compounds will not react with CH3MgBr ?
Ethyl acetate
Acetone
Dimethyl ether
Ethanol
Phenol reacts with dilute nitric acid at normal temperature to form
o-nitrophenol
m-nitrophenol
o-and p-nitropheno
2, 4, 6-trinitrophenol
Which of the following compounds does not give Friedel-Craft's reaction ?
benzene
xylene
nitrobenzene
phenol
Which one is the correct IUPAC name of the following compound?

1-chloro-5-hydroxycydohexane
2-chloro-4-hydroxycyclohexane
3-chloro-3-cyclohexenol
5-hydroxycydohexenyl chloride
