 Multiple Choice Questions
Multiple Choice QuestionsX Y + Z
Y can be obtained by Etard's reaction, Z undergoes disproportionation reaction with concentrated alkali. X could be
![]()
![]()
![]()
![]()
Iodoform reaction is answered by all, except
CH3-CH(OH)-CH2-COOH
CH3CHO
CH3-CH2-OH
CH3-CH2-CH2OH
The distinguishing test between methanoic acid and ethanoic acid is
litmus test
Tollen's test
esterficiation test
sodium bicarbonate test
B.
Tollen's test
Methanoic acid is unique amongst simple carboxylic acids, since it contains a hydrogen atom instead of an alkyl group (HCOOH) i.e it contains both an aldeny dic group and a carboxyl group.

So, like aldehyde, methanoic acid reduces Tollen's reagent to shining silver mirror but ethanoic acid does not give this test.
The formation of cyanohydrins from a ketone is an example of
nucleophilic substitution
nucleophilic addition
electrophilic addition
electrophilic substitution
In the sequence of following reactions
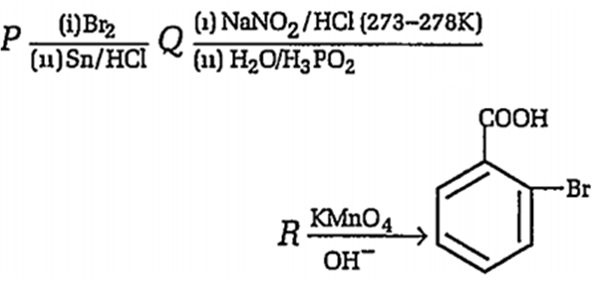
The starting compound P is
o- nitro toluene
m-nitro toluene
o-bromo toluene
p-nitro toluene
Acetic acid is treated with Ca(OH)2 and the product so obtained is subjected to dry distillation. The final product is
propanal
propanone
ethanal
ethanol
An organic compound X is oxidised by using acidified K2Cr2O7 solution. The product obtained reacts with phenyl hydrazine but does not answer silver mirror test. The compound X is,
2-propanol
Ethanal
Ethanol
CH3CH2CH3
