 Multiple Choice Questions
Multiple Choice QuestionsX Y + Z
Y can be obtained by Etard's reaction, Z undergoes disproportionation reaction with concentrated alkali. X could be
![]()
![]()
![]()
![]()
Iodoform reaction is answered by all, except
CH3-CH(OH)-CH2-COOH
CH3CHO
CH3-CH2-OH
CH3-CH2-CH2OH
The distinguishing test between methanoic acid and ethanoic acid is
litmus test
Tollen's test
esterficiation test
sodium bicarbonate test
The formation of cyanohydrins from a ketone is an example of
nucleophilic substitution
nucleophilic addition
electrophilic addition
electrophilic substitution
In the sequence of following reactions
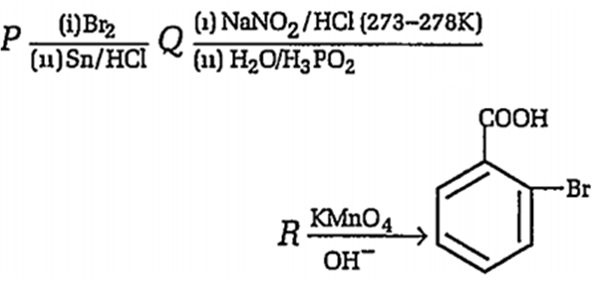
The starting compound P is
o- nitro toluene
m-nitro toluene
o-bromo toluene
p-nitro toluene
Acetic acid is treated with Ca(OH)2 and the product so obtained is subjected to dry distillation. The final product is
propanal
propanone
ethanal
ethanol
An organic compound X is oxidised by using acidified K2Cr2O7 solution. The product obtained reacts with phenyl hydrazine but does not answer silver mirror test. The compound X is,
2-propanol
Ethanal
Ethanol
CH3CH2CH3
Iodoform can be prepared from all, except
propan-2-ol
butan-2-one
propan-1-ol
acetophenone
C.
propan-1-ol
Iodoform test is need for charactersing compounds containing CH3CO or CH3CHOH- which can be easily oxidised to CH3CO group by halogens.

In propan-1-ol (CH3-CH2-CH2OH), CH3-CHOH- group is not present, so it will not give iodoform reaction.
