 Multiple Choice Questions
Multiple Choice QuestionsIn the Lassaigne's test for the detection of nitrogen in an organic compound, the appearance of blue coloured compound is due to
ferric ferricyanide
ferrous ferricyanide
ferric ferrocyanide
ferrous ferrocyanide
Which ofthe following alkanes is optically active?
3-Methylhexane
Propane
2, 3, 4-Trimethylpentane
2-Methylbutane
Optical isomerism is exhibited by (ox= oxalate anion; en= ethylenediamine).
cis-[CrCl2(ox)2]3-
[Co(en)3]3+
trans-[CrCl2(ox)2]3-
[Co(ox) (en)2]+
The IUPAC name of the following molecule is
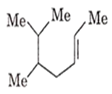
5,6-dimethylhept-2-ene
2,3-dimethylhept-5-ene
5,6-dimethylhept-3-ene
5-iso-propylhex-2-ene
The reagents to carry out the following conversion are
![]()
HgSO4/dil. H2SO4
BH3; H2O2/NaOH
OSO4; HIO4
NaNH2/ CH3I; HgSO4/ dil.H2SO4
The well-known compounds, (+)- lactic acid and (-)- lactic acid, have the same molecular formula, C3H6O3. The correct relationship between them is:
constitutional isomerism
geometrical isomerism
identicalness
optical isomerism
Which one of the following characteristics belong to an electrophile?
It is any species having electron deficiency which reacts at an electron-rich C-centre
It is any species having electron enrichment, that reacts at an electron-deficient C-centre
It is cationic in nature
It is anionic in nature
Among the following structures the one which is not a resonating structure of others is
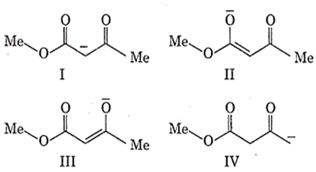
I
II
III
IV
