 Multiple Choice Questions
Multiple Choice QuestionsAmong the following carbocations:
Ph2C+CH2Me (I), PhCH2CH2CH+Ph (II), Ph2CHCH+Me (III) and Ph2C(Me)CH2 (IV), the order of stability is:
IV > II > I > III
I > II > III > IV
II > I > IV > III
I > IV > III > II
Which one of the following will show optical isomerism?
OH-CH2-CO2H
CH3-CH(OH)-CO2H
(CH3)2-CH-CO2H
(CH3)2-C(Cl)-CO2H
Among the following statements about the molecules X and Y, the one(s) which correct is (are)
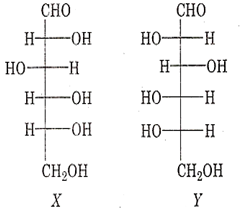
X and Y are diastereomers
X and Y are enantiomers
X and Y are both aldohexoses
X is a D-sugar and Y is an L-sugar
 Short Answer Type
Short Answer Type Multiple Choice Questions
Multiple Choice QuestionsThe two structures written below represent
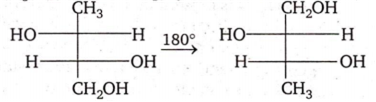
pair of diastereomers
pair of enantiomers
same molecule
both are optically inactive
A compound is formed by substitution of two chlorine for two hydrogens in propane. The number of possible isomeric compounds is
4
3
2
5
1 mole of methyl amine on reaction with nitrous acid gives at NTP
1.0 L of nitrogen
22.4 L of nitrogen
11.2 L of nitrogen
5.6 L of nitrogen
The major product P in the following reaction is
CH3-CH=CH2 P
CH3CH2CH2I
CH3-CH(I)-CH3
CH2(I)-CH=CH2
CH2(I)-CH2(I)
Which of the following will exhibit cis-trans isomerism?
CH2Br-CH2Br
CBr3 - CH3
CHBr = CHBr
CBr2= CH2
