 Long Answer Type
Long Answer TypeGive a brief description of the principles of the following techniques taking an example in each case.
(a) Crystallisation (b) Distillation
(c) Chromatography
Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Estimation of phosphorus:
A known mass of an organic compound is heated with fuming nitric acid whereupon phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate, (NH4)3 PO4.12MoO3, by adding ammonia and ammonium molybdate. Alternatively, phosphoric acid may be precipitated as MgNH4PO4 by adding magnesia mixture which on ignition yields Mg2P2O7.
Estimation of halogens:
Carius method: A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding silver halide (AgX). It is filtered, washed, dried and weighed.
Estimation of sulphur:
A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidised to sulphuric acid. It is precipitated as barium sulphate by adding an excess of barium chloride solution in water. The precipitate is filtered, washed, dried and weighed.
Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
 Short Answer Type
Short Answer TypeIn the organic compound CH2 = CH – CH2 – CH2 – C ≡ CH, the pair of hybridised
orbitals involved in the formation of: C2 – C3 bond is:
(a) sp – sp2
(b) sp – sp3
(c) sp2 – sp3
(d) sp3 – sp3
 Multiple Choice Questions
Multiple Choice QuestionsPresence of nitrogen in which among the following compounds can not be detected by Lassaigne method?
Hydrazine
Aniline
p-toluidine
Picric acid
The hottest region of Bunsen flame shown in the figure below is: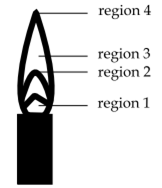
region 2
region 3
region 4
region 4
The distillation technique most suited for separating glycerol from spent-lye in the soap industry is:
fractional distillation
steam distillation
distillation under reduced pressure
distillation under reduced pressure
Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide?
Tertiary butyl chloride
Neopentane
Isohexane
Isohexane
Identify the compound that exhibits tautomerism
2-Butene
Lactic acid
2-Pentanone
2-Pentanone
