 Short Answer Type
Short Answer TypeAccount for the following:
On addition of ozone gas to KI solution, violet vapours are obtained.
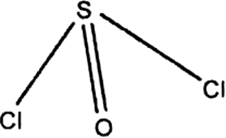
It has a pyramidal structure involving sp3 hybridisation with a lone-pair of electrons as:
So, Lewis basic character is due to the presence of a lone-pair. In addition SOCl2 has also empty d-orbitals which can be used to accept electron pairs and hence it behaves as a Lewis acid.
 Long Answer Type
Long Answer Type