 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss the favourable conditions for the manufacture of (i) ammonia by Haeber's process and (ii) sulphuric acid by contact process.
(i) Favourable conditions for the manufacture of ammonia by Haeber Process:
(i) A high temperature of 400-500°C.
(ii) A high pressure of 200-1000 atoms.
(iii) A catalyst usually Fe + Mo or finely divided iron and Fe3O4 containing small amounts of K2O and Al2O3.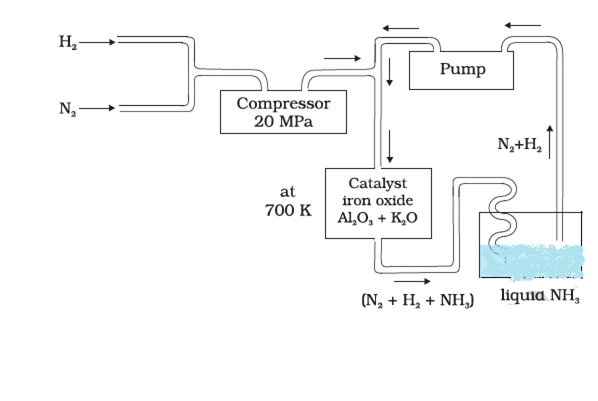
(ii) Favourable conditions for the manufacture of sulphuric acid by contact process:
(i) High concentration of oxygen : Air or oxygen used for the oxidation of sulphur dioxide to sulphur trioxide must be in excess. Furthermore, these gases must be absolutely pure otherwise they will poison the catalyst.
(ii) High pressure: Since the forward reaction proceeds with decrease in volume, therefore, high pressure will favour the reaction. In actual practice, a pressure of about 2 atmospheres is used. This is because gases are acidic and corrosion of the plant occurs at high pressure.
(iii) Low temperature : Since the forward reaction is exothermic, therefore, low temperature will favour the reaction. However, rate of the reaction decreases with decrease in temperature. Therefore, the reaction is carried out at an optimum temperature of 623-723 K.
(iv) Use of catalyst: To increase the rate of a reaction at low temperature, a catalyst is to be used. The commonly used catalysts are platinum, or divanadium pentoxide (V2Os). Since platinum is quite costly and is easily poisoned by arsenic impurities usually present in SO2, therefore, these days, divanadium pentoxide is employed because it is not only cheaper but is also not easily poisoned.
(v) Purity of gases: Purity of gases is another essential conditions for the maximum yield of SO2. The impurities present in the reacting gases act as catalytic poison and thus decrease the efficiency of the catalyst.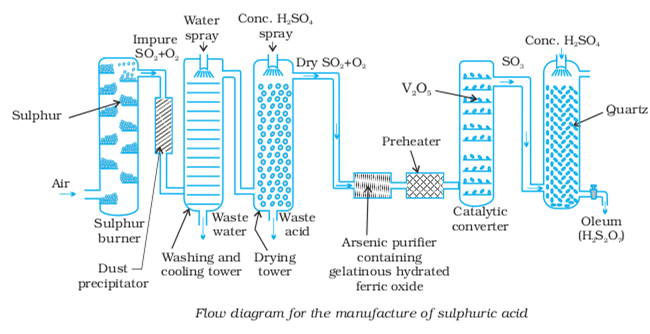
 Short Answer Type
Short Answer Type