 Short Answer Type
Short Answer TypeXenon trioxide is an unstable compound of xenon, it has +6 oxidation state.
Xenon trioxide may be prepared by the hydrolysis of XeF4 or XeF6 .
XeF6 +3H2O ----->XeO3 +6HF
6XeF4 +12H2O----->2XeO3 +4Xe +3O2 +24HF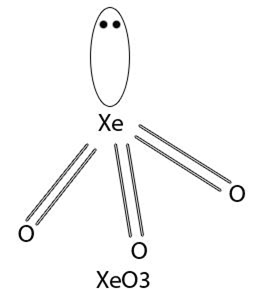
Account for the following:
The boiling points of noble gases increase with the increase in atomic number.
