 Short Answer Type
Short Answer TypeChlorine has empty d-orbital and it acquires excited state at the time of bonding when electron from 3p-orbital are promoted to 3d- orbital.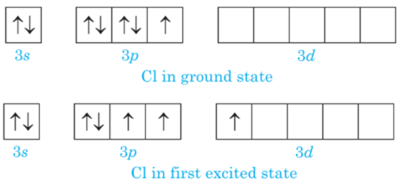
In first excited state chlorine atom can exhibit a covalency of three, hence cannot expand its octetdur to absence of empty d- orbitals in 2nd energy shell.
Hence, it cannot exhibit covalency more than 1therefore FCl3 is not possible.
Account for the following:
The boiling points of noble gases increase with the increase in atomic number.
