 Short Answer Type
Short Answer TypeHow would you account for the following:
Interhalogen compounds are strong oxidising agents.
How would you account for the following:
Sulphur hexafluoride is less reactive than sulphur tetrafluoride.
How would you account for the following:
In the noble gases only xenon forms known chemical compounds.
The shape of SF4 is see saw geometry.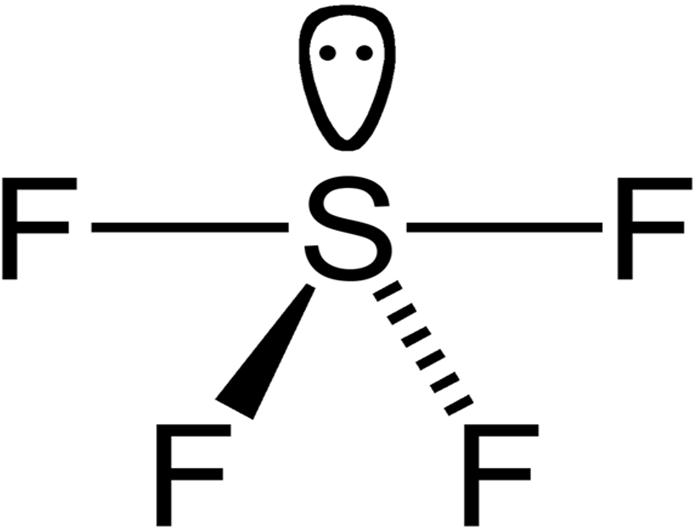
SF6 has an octahedral geometry.

 Long Answer Type
Long Answer TypeDescribe the molecular shapes of the following: (i) SF4, (ii) BrF5, (iii) IF3, (iv) PF5, (v) XeF2.
