 Short Answer Type
Short Answer TypeGive reasons for the following observations:
It is necessary to remove CO when ammonia is prepared by Haber's process.
Account for the following:
(i) Acidic character increases from HF to HI.
(ii) There is a large difference between the melting and boiling points of oxygen and sulphur.
(iii) Nitrogen does not form pentahalide.
 Long Answer Type
Long Answer Type(i) Which allotrope of phosphorus is more reactive and why?
(ii) How the supersonic jet aeroplanes are responsible for the depletion of ozone layers?
(iii) F2 has lower bond dissociation enthalpy than Cl2. Why?
(iv) Which noble gas is used in filling balloons for meteorological observations?
(v) Complete the equation: XeF2 + PF5 →
i) White phosphorus is most reactive of all the allotropes of phosphorus because it is unstable due to the angular strain on P4 molecule with the bond angle of 60°.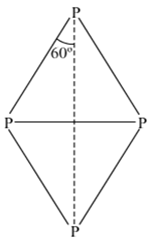
(ii) Nitrogen oxide emitted from the exhausts of supersonic jet aeroplanes readily combine with ozone to form nitrogen dioxide and diatomic oxygen.
NO(g) + O3(g) →NO2(g) +O2(g)
Since supersonic jets fly in the stratosphere near the ozone layer, they are responsible for the depletion of ozone layer.
(iii) The size of a fluorine atom is very small as compared to a chlorine atom. Therefore, the repulsion between electrons in the outer most shell of the two atoms in a fluorine molecule is much greater than that in a chlorine molecule. Hence, it requires less energy to break up the fluorine molecule, making its bond dissociation energy lesser than that of chlorine molecule.
(iv) Helium, being light, non-inflammable and unreactive, is used for filling of balloons for metrological observations.
(v) XeF2 + PF5→ [XF]++[PF6]-
 Short Answer Type
Short Answer TypeComplete the following chemical equations:
(i) Ca3P2 + H2O -->
(ii) Cu + H2SO4(conc.)-->
Arrange the following in the order of the property indicated by each set:
(i) HF, HCl, HBr, HI - increasing bond dissociation enthalpy.
(ii) H2O, H2S, H2Se, H2Te- increasing acidic character.
Account for the following:
(i) PCl5 is more covalent than PCl3.
(ii) Iron on reaction with HCl forms FeCl2 and not FeCl3.
