 Short Answer Type
Short Answer TypeAmong the hydrides of Group-15 elements, which have the
When concentrated sulphuric acid was added to an unknown salt present in a test tube a brown gas (A) was involved. This gas intensified when copper turnings were added to this test tube. On cooling, the gas (A) changed into a colourless solid (B).
(i) Identify (A) and (B)
(ii) Write the structures of (A) and (B)
(iii) Why does gas (A) change to solid on cooling
(i) A is NO2 gas (because NO3- salt reacts with conc. H2SO4 to give NO2 gas which is brown). (B) is N2O4
(ii)
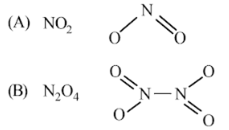
(iii) NO2 (A) is an odd electron-molecule. Thus, to become stable, it decreases to give N2O4 which is a colourless solid.
 Multiple Choice Questions
Multiple Choice QuestionsOxidation number of nitrogen in which among the oxides of nitrogen is the lowest?
Nitric oxide
Nitrous oxide
Nitrogen dioxide
Nitrogen trioxide
The reaction of zinc with dilute and concentrated nitric acid, respectively, produces:
NO2 and NO
NO and N2O
NO2 and N2O
NO2 and N2O
