Multiple Choice Questions
Multiple Choice QuestionsOxyacids of phosphorus and the starting materials for their preparation are given below.
| Oxyacid | Materials for preparation |
| A. H3PO2 | i. Red P + alkali |
| B. H3PO3 | ii. P4O10 + H2O |
| C. H3PO4 | iii. P2O3 + H2O |
| D. H4P2O6 | iv. White P + alkali |
Choose the correct answer from the codes given below
A - iv; B - iii; C - ii; D - i
A - i; B - iii; C - ii; D - iv
A - iv; B - iii; C - i; D - ii
A - ii; B - iii; C - i; D - iv
The reaction takes place in two steps as
Identify the reaction intermediate.
NO2Cl (g)
NO2(g)
Cl2(g)
Cl(g)
Which one of the following oxides of nitrogen dimerises into colourless is solid/ liquid on cooling?
N2O
NO
N2O3
NO2
Halogens exist in -1,+ 1, + 3, + 5 and 47 oxidation states. The halogen that exists only in -1 state is
F
Cl
Br
I
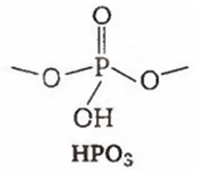
Among the oxyacids of phosphorus, the dibasic acid is
H3PO3
H4P2O7
H2PO2
HPO3
A.
H3PO3
H3PO3 is a dibasic acid as it contains two replaceable hydrogen atoms.
(a) 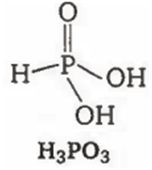
(b) 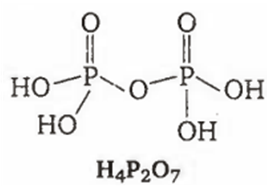
(c) 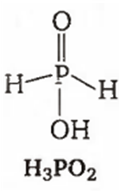
(d)