 Multiple Choice Questions
Multiple Choice Questions(+)-2-chloro-2-phenylethane in toluene racemises slowly in the presence of small amount of SbCl2, due to the formation of
carbanion
carbene
free-radical
carbocation
Correct pair of compounds which gives blue colouration/precipitate and white precipitate, respectively, when their Lassaigne's test is separately done is
NH2NH2, HCl and ClCH2COOH
NH2CSNH2 and PhCH2Cl
NH2NH2, COOH and NH2CONH2

2-methylpropane on monochlorination under photochemical condition give
2-chloro-2-methylpropane as major product
(1 : 1) mixture of 1-chloro-2-methylpropane and 2-chloro-2-methyl propane
1-chloro-2-methyl propane as a major product
(1 : 9) mixture of 1-chloro-2-methylpropane and 2-chloro-2-methylpropane
An optically active compound having molecular formula C2H16 on ozonolysis gives acetone as one of the products. The structure of the compound is




Among the following statements about the molecules X and Y, the one(s) which correct is (are)
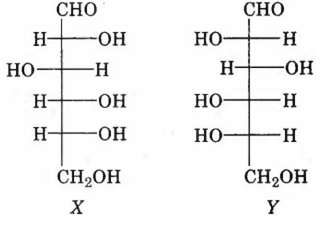
X and Y are diastereomers
X and Y are enantiomers
X and Y are both aldohexoses
Xis a D-sugar and Y is an L-sugar
Amongst the following, the one which can exist in free state as stable compound is
C7H9O
C8H12O
C6H12O
C10H17O
 Short Answer Type
Short Answer TypeCompound A treated with NaNH2 followed by CH3CH2Br gave compound B. Partial hydrogenation of compound B produced compound C, which on ozonolysis gave a carbonyl compound D, (C3H6O). Compound D did not respond to iodoform test with I2/KI and NaOH. Find out the structures of A, B, C and D.
