 Multiple Choice Questions
Multiple Choice QuestionsWhich statement is not correct for ortho and para hydrogen?
They have different boiling points
Ortho-form is more stable than para-form
They differ in their nuclear spin
The ratio of ortho to para hydrogen changes with change in temperature
In the IUPAC system, PhCH2CH2CO2H is named as
3-phenylpropanoic acid
benzylacetic acid
carboxyethylbenzene
2-phenylpropanoic acid
The correct order of acid strengths of benzoic acid (X), peroxybenzoic acid (Y) and p-nitrobenzoic acid (Z) is
Y > Z > X
Z > Y > X
Z > X > Y
Y > X > Z
Choose the correct statement(s) among the following.
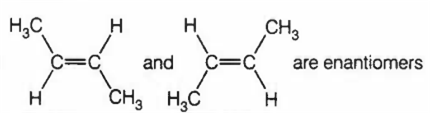
CH3CHO on reaction with HCN gives racemic mixture

CH3-CH=NOH shows geometrical isomerism
Optical isomerism is exhibited by (ox= oxalate anion; en= ethylenediamine).
cis-[CrCl2(ox)2]3-
[Co(en)3]3+
trans-[CrCl2(ox)2]3-
[Co(ox)(en)2]+
In a mixture, two enantiomers are found to be present in 85% and 15% respectively. The enantiomeric excess (ee) is
85%
15%
70%
60%
Among the following structures the one which is not a resonating structure of others is

I
II
III
IV
In the Lassaigne's test for the detection of nitrogen in an organic compound, the appearance of blue coloured compound is due to
ferric ferricyanide
ferrous ferricyanide
ferric ferrocyanide
ferrous ferrocyanide
C.
ferric ferrocyanide
To a part of sodium extract, FesO4 solution is added and the contents are warmed. A few drops of FeCl3 solution are then added and resulting solution is acidified with cone. HCl. The appearance of bluish green or Prussian blue colouration confirms the presence of nitrogen. The reactions that occur during this test are as
2NaCN + FeSO4 → Na2SO4 + Fe(CN)2
Fe(CN)2 + 4NaCN → Na4[Fe(CN)6]
3Na4[Fe(CN)6] + 4FeCl3 → Fe4[Fe(CN)6]3 + 12NaCl
The IUPAC name of the compound X is
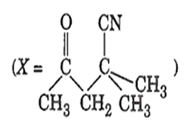
4-cyano-4-methyl-2-oxopentane
2-cyano-2-methyl- 4-oxopentane
2, 2-dimethyl-4-oxopentanenitrile
4-cyano-4-methyl-2-pentanone
