 Multiple Choice Questions
Multiple Choice QuestionsIf average velocity of a sample of gas molecules at 300 K is 5 cm s-1, what is RMS velocity of same sample of gas molecules at the same temperature? (Given, α: u: v = 1:1.224: 1.127)
6.112 cm/s
4.605 cm/s
4.085 cm/s
5.430 cm/s
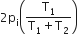
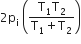
Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T2. The final pressure pf is: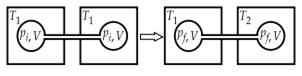
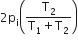

The intermolecular interaction that is dependent on the inverse cube of the distance between the molecule is:
ion-ion interaction
ion-dipole interaction
London force
London force
If Z is a compressibility factor, Vander Waal's equation at low pressure can be written as
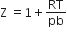
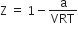
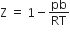
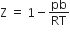
For the gaseous state, if most probable speed is denoted by C*, average speed by C and mean square speed by C, then for a large number of molecules the ratios of these speeds are:
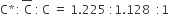
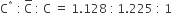
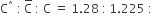
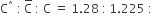
'a' and 'b' are van der Waals constants for gases. Chlorine is more easily liquefied than ethane because :
a and b for Cl2 > a and b for C2H6
a and b for Cl2 < a and b for C2H6
a and Cl2 < a for C2H6 but b for Cl2 > b for C2H6
a and Cl2 < a for C2H6 but b for Cl2 > b for C2H6
If 10–4 dm3 of water is introduced into a 1.0 dm3 flask at 300 K, how many moles of water are in the vapour phase when equilibrium is established?(Given: Vapour pressure of H2O at 300 K is 3170 Pa; R = 8.314 J K–1 mol–1)
5.56 x 10-3 mol
1.53 x 10-2 mol
4.46 x 10-2 mol
4.46 x 10-2 mol
Assuming that water vapour is an ideal gas, the internal energy change(∆U) when 1 mol of water is vapourised at 1 bar pressure and 100°C, (Given: Molar enthalpy of vapourisation of water at 1 bar and 373 K = 41 kJ mol-1 and R = 8.3 J mol–1K–1 will be) –
4.100 kJ mol–1
3.7904 kJ mol–1
37.904 kJ mol–1
37.904 kJ mol–1
