 Multiple Choice Questions
Multiple Choice QuestionsPhosphorus pentachloride dissociates as follows, in a closed reaction vessel,
PCl5 (g) ⇌ PCl3(g) + cl2(g)
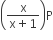
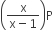
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be
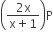

The formation of the oxide ion O2-(g) requires first an exothermic and then an endothermic step as shown below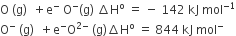
Oxygen is more electronegative
O- ion has comparatively larger size than oxygen atom
O- ion will tend to resist the addition of another electron
O- ion will tend to resist the addition of another electron
As the temperature is raised from 20°C to 40°C, the average kinetic energy of neon atoms changes by a factor of which of the following?
1/2
2
313/293
313/293
In Vander Waals equation of state of the gas law, the constant ‘b’ is a measure of
intermolecular repulsions
intermolecular collisions per unit volume
Volume occupied by the molecules
Volume occupied by the molecules
An ideal gas expands in volume from 1×10-3 m3 to 1×10-2 m3 at 300 K against a constant pressure of 1×105 Nm-2. The work done is
-900 J
900 J
2780 J
2780 J
For same mass of two different ideal gases of molecular weights M1 and M2, Plots of log V vs log p at a given constant temperature are shown. Identify the correct option.
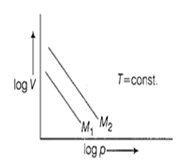
M1 > M2
M1 = M2
M1 < M2
Can be predicted only if temeperature is known
Which of the following has the dimension if [ML0T-2]?
Coefficient of viscosity
Surface tension
Vapour pressure
Kinetic energy
The boiling points of HF, HCl, HBr and HI follow the order
HF> HCl > HBr > HI
HF> HI> HBr > HCl
HI> HBr > HCl> H
HCl > HF> HBr > HI
At a certain temperature, the value of the slope of the plot of osmotic pressure () against concentration (C in molL-1) of a certain polymer solution is 291R. The temperature at which osmotic pressure is measured, is (R is gas constant)
271°C
18°C
564 K
18 K
For one mole of an ideal gas, the slope of V vs. T curve at constant pressure of 2 atm is X L mol-1 K-1.The value of the ideal universal gas constant 'R' in terms of X is
X L atm mol-1 K-1
x/2 L atm mol-1 K-1
2X L atm mol-1 K-1
2X atm L-1 mol-1 K-1
