 Multiple Choice Questions
Multiple Choice QuestionsIn Williamson's synthesis, ethoxyethane is prepared by :
passing ethanol over heated alumina
heating sodium ethoxide with ethyl bromide
treating ethyl alcohol with excess of H2SO4 at 430-440 K
heating ethanol with dry Ag2O
The correct order of reactivity of phenyl Magnesium bromide with the following compound is:
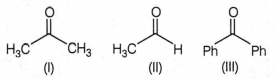
(II)> (III)> (I)
(I)> (III)> (II)
(II)> (I)> (III)
(I)> (II)> (III)
The alcohol that produces turbidity immediately with ZnCL2 + conc. HCl at room temperature:
1- hydroxybutane
2- hydroxybutane
2- hydroxy -2- methyl propane
1- hydroxy -2- methyl propane
The compound which reacts faster with Lucas reagent is
butan-1-ol
butan-2-ol
2-methyl-propan-1-ol
2-methyl propan-2-ol
In the given reaction ,
R-OH + HX RX + H2O
the order of reactivity of alcohols is:
tertiary < secondary < primary
tertiary > secondary > primary
tertiary < secondary > primary
secondary > primary > tertiary
B.
tertiary > secondary > primary
Reactions of alcohols involving cleavage of C-OH bond follow the reactivity order is tertiary > secondary > primary. With reference to the stability of carbocation intermediate.
A compound is soluble in conc. H2SO4 . It does not decolourise bromine in CCL4 but in oxidised by chromic anhydride in aqueous H2SO4 with 2s , turning orange solution to blue green , afterward opaque. The original solution contains :
a primary alcohol
a secondary alcohol
a tertiary alcohol
an ether
Which of following is the correct order of increasing reactivity?
RCOOR' < RCOCl < RCONH2
RCOOR' < RCONH2 < RCOCl
RCOCl < RCONH2 < RCOOR'
RCONH2 < RCOOR' < RCOCl
