 Multiple Choice Questions
Multiple Choice QuestionsIn Williamson's synthesis, ethoxyethane is prepared by :
passing ethanol over heated alumina
heating sodium ethoxide with ethyl bromide
treating ethyl alcohol with excess of H2SO4 at 430-440 K
heating ethanol with dry Ag2O
The correct order of reactivity of phenyl Magnesium bromide with the following compound is:
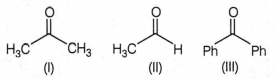
(II)> (III)> (I)
(I)> (III)> (II)
(II)> (I)> (III)
(I)> (II)> (III)
The alcohol that produces turbidity immediately with ZnCL2 + conc. HCl at room temperature:
1- hydroxybutane
2- hydroxybutane
2- hydroxy -2- methyl propane
1- hydroxy -2- methyl propane
The compound which reacts faster with Lucas reagent is
butan-1-ol
butan-2-ol
2-methyl-propan-1-ol
2-methyl propan-2-ol
In the given reaction ,
R-OH + HX RX + H2O
the order of reactivity of alcohols is:
tertiary < secondary < primary
tertiary > secondary > primary
tertiary < secondary > primary
secondary > primary > tertiary
A compound is soluble in conc. H2SO4 . It does not decolourise bromine in CCL4 but in oxidised by chromic anhydride in aqueous H2SO4 with 2s , turning orange solution to blue green , afterward opaque. The original solution contains :
a primary alcohol
a secondary alcohol
a tertiary alcohol
an ether
A.
a primary alcohol
The given compounds is soluble in H2SO4 the compound should be either alcohol or ether. It does not decolourise bromine, which shows that compound does not contain double bond . Compounds is oxidised by chromic anhydride in aqueous H2SO4 i.e. compound does not contain ether group. As the oxidation is very fast, the compound (alcohol) may be a primary alcohol.
Which of following is the correct order of increasing reactivity?
RCOOR' < RCOCl < RCONH2
RCOOR' < RCONH2 < RCOCl
RCOCl < RCONH2 < RCOOR'
RCONH2 < RCOOR' < RCOCl
