 Multiple Choice Questions
Multiple Choice QuestionsIn the conversion of limestone to lime, CaCO3(s) → CaO(s) + CO2(g) the values of ∆Hº and ∆Sº are + 179.1 kJ mol–1 and 160.2 J/K respectively at 298K and 1 bar. Assuming that ∆Hº and ∆Sº do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is
1008 K
1200 K
845 K
845 K
The standard enthalpy of formation (∆fHo) at 298 K for methane, CH4(g), is –74.8 kJ mol–1. The additional information required to determine the average energy for C – H bond formation would be
the dissociation energy of H2 and enthalpy of sublimation of carbon
latent heat of vapourization of methane
the first four ionization energies of carbon and electron gain enthalpy of hydrogen
the first four ionization energies of carbon and electron gain enthalpy of hydrogen
The enthalpy changes for the following processes are listed below:
Cl2(g) = 2Cl(g), 242.3 kJ mol–1
I2(g) = 2I(g), 151.0 kJ mol–1
ICl(g) = I(g) + Cl(g), 211.3 kJ mol–1
I2(s) = I2(g), 62.76 kJ mol–1
Given that the standard states for iodine and chlorine are I2(s) and Cl2(g), the standard enthalpy of formation for ICl(g) is
–14.6 kJ mol–1
–16.8 kJ mol–1
+16.8 kJ mol–1
+16.8 kJ mol–1
(∆H −∆U) for the formation of carbon monoxide (CO) from its elements at 298 K is
(R = 8.314 J K–1 mol–1)
–1238.78 J mol–1
1238.78 J mol–1
–2477.57 J mol–1
–2477.57 J mol–1
Consider the reaction: N2 +3H2 → 2NH3 carried out at constant temperature and pressure. If ∆H and ∆U are the enthalpy and internal energy changes for the reaction, which of the following expressions is true?
∆H = 0
∆H = ∆U
∆H < ∆U
∆H < ∆U
Which one of the following statements is NOT true about the effect of an increase in temperature on the distribution of molecular speeds in a gas?
The most probable speed increases
The fraction of the molecules with the most probable speed increases
The distribution becomes broader
The distribution becomes broader
If the bond dissociation energies of XY, X2 and Y2 (all diatomic molecules) are in the ratio of 1:1:0.5 and ∆f H for the formation of XY is -200 kJ mole-1. The bond dissociation energy of X2 will be
100 kJ mol-1
800 kJ mol-1
300 kJ mol-1
300 kJ mol-1
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283 kJ mol-1 respectively. The enthalpy of formation of carbon monoxide per mole is
110.5 kJ
-110.5 kJ
-676.5 kJ
-676.5 kJ
Which of the following lines correctly show the temperature dependence of equilibrium constant K, for an exothermic reaction?
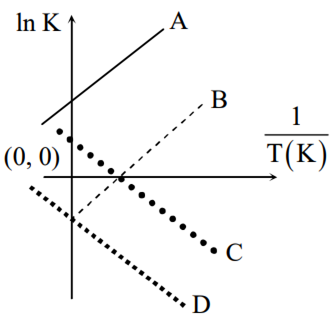
A and D
A and B
B and C
C and D
The combustion of benzene (l) gives CO2(g) and H2O(l). Given that heat of combustion of benzene at constant volume is –3263.9 kJ mol–1 at 250C; the heat of combustion (in kJ mol–1) of benzene at constant pressure will be (R = 8.314 JK–1 mol–1)
–3267.6
4152.6
–452.46
3260
