 Multiple Choice Questions
Multiple Choice QuestionsEnthalpy (H) is equal to
internal energy (E)
product of pressure (p) and volume (V) of gas
internal energy (E)+ pV
work (W) done by a system
Minimum work is obtained when 1 kg of...gas expanded under 500 kPa to 200 kPa pressure at 0°C
chlorine
oxygen
nitrogen
methane
Which one of the following represents the graph between log p (on Y-axis) and 1/T on X-axis)? (p = vapour pressure of a liquid, T = absolute temperature).
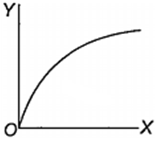
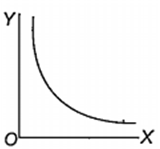
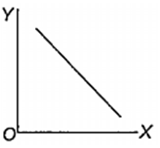
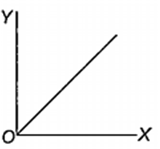
When 10 g of methane is completely burnt in oxygen, the heat evolved is 560 kJ. What is the heat of combustion (in kJ mol-1) of methane?
-1120
-968
-896
-560
C.
-896
Calculate the heat of combustion (in kJ) of methane from the following data :
(i) C(graphite) + 2H2 (g) → CH4 (g) ; H = -74.8 kJ
(ii) C(graphite) + O2 (g) → CO2 (g) ; H = -393.5 kJ
(iii) H2 (g) + 1/2 O2 (g) → H2O (l) ; H = -286.2 kJ
-891.1
-816.3
-965.9
-1040.7
Which of the following is an endothermic reaction?
N2 (g) + 3H2 (g) - 92 kJ → 2NH3 (g)
N2 (g) + O2 (g) + 180.8 kJ → 2NO (g)
H2 (g) + Cl2 (g) → 2HCl (g) + 184.6 kJ
C (graphite) + 2H2 (g) → CH2 (g) + 74.8 kJ
Which of the following is not correct?
3O2 2O3 ; H = -284.5 kJ
Ozone undergoes addition reaction with unsaturated carbon compounds
Sodium thuosulphate reacts with I2 to form sodium tetrathionate and sodium iodide.
Ozone oxidises lead sulphide to lead sulphate.
