 Multiple Choice Questions
Multiple Choice QuestionsPredict the correct order among the following.
long pair-lone pair> bond pair-bond pair> lone pair>bond pair
bond pair-bond pair> lone pair-bond pair > lone pair -lone pair
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
lone pair -bond pair > bond pair- bond pair> lone pair -lone pair
The pair of an electron in the given carbanion, is present in which orbital?
is present in which orbital?
sp3
sp2
sp
sp
Consider the molecules CH4, NH3 and H2O. Which of the given statement is false?
The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4.
The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3.
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
Which of the following species contains an equal number of σ and Π bond?
HCO3-
XeO4
(CN)2
(CN)2
Consider the following compounds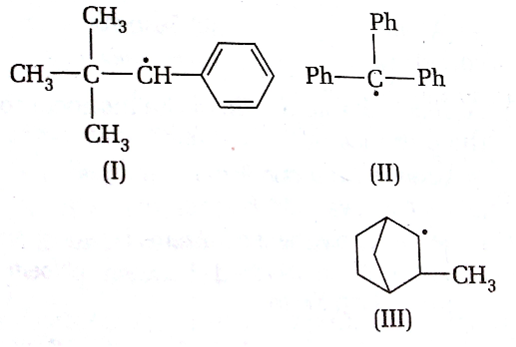
Hyperconjugation occurs in
I only
II only
III only
III only
The enolic form of ethyl acetoacetate as below has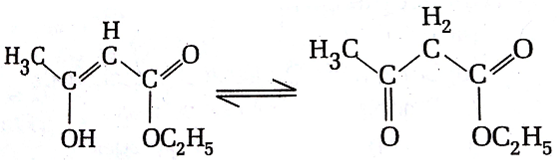
18 sigma bonds and 2 pi-bond
16 sigma bonds and 1 pi-bond
9 sigma bonds and 2 pi-bond
9 sigma bonds and 2 pi-bond
The pair of compounds that can exist together is
FeCl3.SnCl2
HgCl2,SnCl2
FeCl2,SnCl2
FeCl2,SnCl2
