 Multiple Choice Questions
Multiple Choice QuestionsThe energy released when 6 moles of octane is burnt in air will be [Given, ΔHf for CO2 (g). H2O(g) and C8H18 (l), respectively are -490, -240 and +160J/mol]
-37.4 kJ
-20 kJ
-6.2 kJ
-35.5 kJ
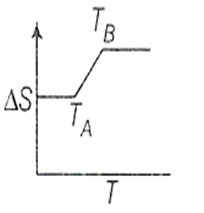
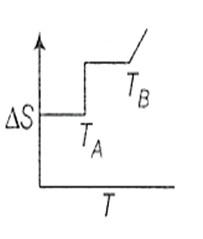
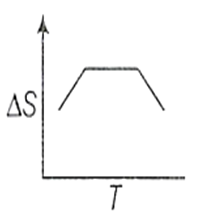
If for a given substance, melting point is TB and freezing point is TA then correct variation of entropy by graph between entropy change and temperature is

Which of the following is the increasing order of enthalpy of vaporization?
NH3, PH3, AsH3
AsH3, PH3 NH3
NH3, AsH3, PH3
PH3, AsH3, NH3
For the process to occur under adiabatic conditions, the correct condition is
T = 0
p = 0
q = 0
W = 0
O2(g) O3(g); Kp for this reaction is 2.47 x 10-29. At 298 K, for conversion of oxygen to ozone will be
100 kJ mol-1
150 kJ mol-1
163 kJ mol-1
2303 kJ mol-1
According to the first law of thermodynamics which of the following quantities represents the change in a state function?
qrev
qrev - Wrev
qrev + Wrev
What is the H of the reaction?
CH2Cl2 (g) C (g) + 2H (g) + 2Cl (g)
The average bond energies of C-Cl bond and C-H bond are 416 kJ and 325 kJ mol-1 respectively.
1482 kJ
1482 J
1492 kJ
1492 J
The heat of formation of CO(g) and CO2 (g)are H = -110 and H = - 393 kJ mol-1 respectively. What is the heat of reaction (H) (in kJ mol-1) for the following reaction?
CO (g) + (g) CO2(g)
-504
-142.5
-283
504
