 Multiple Choice Questions
Multiple Choice QuestionsThe order of decreasing reactivity towards an electrophilic reagent, for the following:
i) Benzene
ii) Toluene
iii) Chlorobenzene
iv) Phenol
would be:
(i) > (ii) > (iii) > (iv)
(ii) > (iv) > (i) > (iii)
(iv) > (iii) > (ii) >(i)
(iv) > (iii) > (ii) >(i)
The major organic product in the reaction,
CH3 -O- CH(CH3)2 + HI → Product is:
CH3OH + (CH3)2 + CHI
ICH2OCH(CH3)2
CH3O CI(CH3)2
CH3O CI(CH3)2
Which one is the correct order of acidity?
CH2= CH2> CH3– CH = CH2> CH3– C ≡ CH > CH ≡ CH
CH ≡ CH > CH3– C ≡ CH > CH2= CH2> CH3– CH3
CH ≡ CH > CH2= CH2> CH3– C ≡ CH >CH3– CH3
CH ≡ CH > CH2= CH2> CH3– C ≡ CH >CH3– CH3
The heating of phenyl-methyl ethers with HI produces.
Ethyl chlorides
Iodobenzene
Phenol
Phenol
The compound A on treatment with Na gives B, and with PCl5 gives C. B and C react together to give diethyl ether. A, B and C are in the order
C2H5OH, C2H6, C2H5Cl
C2H5OH, C2H5Cl, C2H5ONa
C2H5OH, C2H5ONa, C2H5Cl
C2H5Cl, C2H6, C2H5OH
In the reaction,
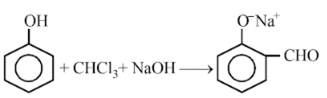
The electrophile involved is
Dichloromethyl cation (C+HCl2)
Formyl cation (C+HO)
Dichlorocarbene (:CCl2)
Dichloromethyl anion (C-HCl2)
Aqueous 10% NaHCO3 solution is used as a reagent for identifying 'A'. Which of the following compounds yield 'A' on hydrolysis?
CH3COOC2H5
C2H5-COO-C2H5
CH3CHO
CH3CH2OH
