 Multiple Choice Questions
Multiple Choice QuestionsA organic compound 'A' on treatment with NH3 gives 'B' which on heating gives 'C', 'C" when treated with Br2 in the presence of KOH produces ethylamine. Compound ' A' is
CH3COOH
CH3CH2CH2COOH
CH3(CH3)CHCOOH
CH3(CH3)CHCOOH
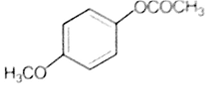
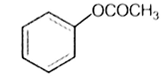
Which one of the following esters gets hydrolysed most easily under alkaline conditions?
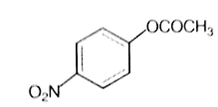

The oxidation of benzene by V2O5 in the presence of air produces
benzoic anhydride
maleic anhydride
benzoic acid
benzoic acid
Acetamide is treated with the following reagents separately. Which one of these would yield methylamine?
NaOH - Br2
Sodalime
Hot conc. H2SO4
Hot conc. H2SO4
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
CH3COOCH3
CH3CONH2
CH3COOCOCH3
CH3COOCOCH3
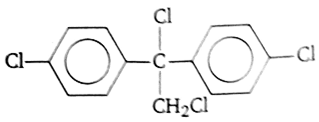
Trichloroacetaldehyde, CCl3CHO reacts with chlorobenzene in presence of sulphuric acid and produces


Propionic acid with Br2-P yields a dibromo product. Its structure would be
CH2Br - CHBr -COOH
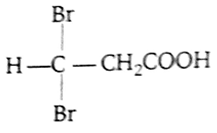
CH2-Br-CH2-COBr
CH2-Br-CH2-COBr
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure
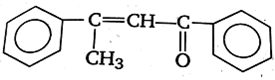
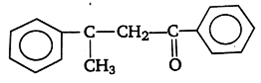
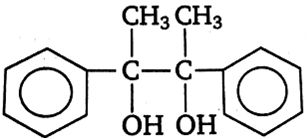
![]()
The relative reactivities of acyl compounds towards of nucleophilic substitution are in the order of
Acyl chloride> Acid anhydride> Ester> Amide
Ester > Acyl chloride> Amide > Acid anhydride
Acid anhydride > Amide > Ester> Acyl chloride
Acyl chloride > Ester > Acid anhydride > Amide
