Multiple Choice Questions
Multiple Choice QuestionsIn nth orbit of hydrogen atom energy , then energy required to displace electrons from n = 1 to n = 2 is
- 10.2 eV
3.4 eV
- 13.6 eV
1.51 eV
The minimum energy to ionize an atom is the energy required to
add one electron to the atom
excite the atom from its ground state to its excited state
remove one outer most electron from the atom
remove one inner most electron from the atom
In hydrogen atom, the electron in a given orbit has total energy -1.5 eV. The potential energy is
1.5 eV
- 1.5 eV
3.0 eV
- 3.0 eV
The wavelength of first Lyman series in the transition from n = 2 to n = 1 is
110 nm
120 nm
122 nm
125 nm
λ1, λ2, λ3 are de-Broglie wavelengths of electron, proton and - particle. If they are accelerated by same potential, then
λ1 < λ2 < λ3
λ1 >λ2 > λ3
λ1 < λ2 > λ3
λ1 > λ2 < λ3
Which energy state of triply ionized beryllium Be3+ has the same orbital radius as that of ground state of hydrogen atom ?
n = 8 state
n = 5 state
n = 4 state
n = 2 state
In Bohr's model of hydrogen atom, the electron circulates around the nucleus in a path of radius 5 x 10-11 m at a frequency of 6.8 x 1015 Hz. The value of magnetic field and equivalent magnetic moment will be
15.4 T and 8.9 x 10-24 A-m2
13.4 T and 8.9 x 10-24 A-m2
18.4 T and 9.8 x 10-24 A-m2
13.4 T and 9.8 x 10-24 A-m2
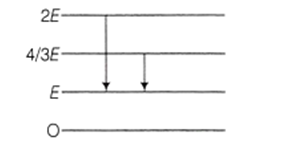
The energy levels of a certain atom are represented in the figure given below. During the transition from 2E to E level, a photon of wavelength is emitted. The wavelength of photon produced during transition from 4 / 3E level to E will be