 Multiple Choice Questions
Multiple Choice QuestionsRatio of longest wavelength corresponding to Lyman and Balmer series in hydrogen spectrum is
5/27
3/23
7/29
7/29
A.
5/27
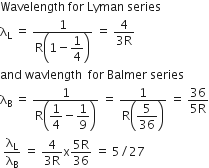
Electrons used in an electron microscope are accelerated by a voltage of 25 kV. If the voltage is increased to 100 kV then the de - Broglie wavelength associated with the electrons would
decrease by 2 times
decrease by 4 times
increase by 4 times
increase by 4 times
The wavelength of the first line of Layman series for the hydrogen atom is equal to that of the second line of Balmer series for a hydrogen-like an ion. The atomic number Z of hydrogen-like ion is
4
1
2
2
In the spectrum of hydrogen, the ratio of the longest wavelength in the Lyman series to the longest wavelength in the Balmer series is
4/9
9/4
27/5
27/5
The energy of a hydrogen atom in the ground state is -13.6 eV. The energy of a He+ ion in the first excited state will be
-13.6 eV
-27.2 eV
-54.4eV
-54.4eV
The potential difference that must be applied to stop the fastest photoelectrons emitted by a nickel surface, having work function 5.01 eV, when ultraviolet light of 200 nm falls on it, must be
2.4 V
-1.2 V
-2.4 V
-2.4 V
The electron in the hydrogen atom jumps from excited state (n=3) to its ground state (n=1) and the photons thus emitted irradiate a photosensitive material. If the work functions of the material is 5.1 eV, the stopping potential is estimated to be (the energy of the electron is nth state En = -13.6/n2 eV)
5.1 V
12.1 V
17.2 V
17.2 V
In a Rutherford scattering experiment when a projectile of charge Z1 and mass M1 approaches a target nucleus of charge Z2 and mass M2, the distance of closest approach is ro.The energy of the projectile is
directly proportional to M1 x M2
directly proportional to Z1Z2
inversely proportional to Z1
inversely proportional to Z1
The ionisation energy of the electron in the hydrogen atom in its ground state is 13.6 eV. The atoms are excited to higher energy levels to emit radiations of 6 wavelengths. Maximum wavelength of emitted radiation corresponds to the transition between
n = 3 to n =2 states
n = 3 to n = 1 states
n = 2 to n = 1 states
n = 2 to n = 1 states
The total energy of an electron in the ground state of a hydrogen atom is -13.6 eV. The kinetic energy of an electron in the first excited state is:
3.4 eV
6.8 eV
13.6 eV
13.6 eV
