 Multiple Choice Questions
Multiple Choice QuestionsThe rate of constant of the reaction A → B is 0.6 x 10-3 mole per second. If the concentration of A is 5 M then concentration of B after 20 min is
1.08 M
3.60 M
0.36 M
0.36 M
The half -life of a substance in a certain enzyme catalysed reaction is 138 s. The time required for the concentration of the substance to fall from 1.28 mg L-1 is
414 s
552 s
690 s
690 s
The rate of reaction 2N2O3 → 4 NO2 + O2 can be written in three ways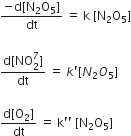
The relationship between k and k' and between k and k'' are
k' = 2k ; k' = k
k' = 2k; k" = k/2
k' = 2k; k' = 2k
k' = 2k; k' = 2k
The unit of rate constant for a zero order reaction is
mol L-1 s-1
L mol-1 s-1
L2 mol-2 s-1
L2 mol-2 s-1
For the reaction
the value of the rate of disappearance of N2O5 is given as
6.25 x 10-3 mol L-1s-1 and 6.25 x 10-3 mol L-1 s-1
1.25 x 10-2 mol L-1s-1 and 6.25 x 10-3 mol L-1 s-1
6.25 x 10-3 mol L-1s-1 and 3.125 x 10-3 mol L-1 s-1
6.25 x 10-3 mol L-1s-1 and 3.125 x 10-3 mol L-1 s-1
For an endothermic reaction, energy of activation is Ea and enthalpy of reaction is ΔH (both of these in kJ/mol). Minimum value of Ea will be
less than ΔH
equal to ΔH
more than ΔH
more than ΔH
During the kinetic study of the reaction, 2A + B --> C+ D, following results were obtained
|
|
[A]/mol L- |
[B]/ mol L- |
Initial rate of formation of D/ mol L- min- |
|
I |
0.1 |
0.1 |
6.0 x 10-3 |
|
II |
0.3 |
0.2 |
7.2 x 10-2 |
|
III |
0.3 |
0.4 |
2.88 x 10-1 |
|
IV |
0.4 |
0.1 |
2.40 x10-2 |
rate = k[A]2[B]
rate = k [A][B]
rate = k[A]2[B]2
rate = k[A]2[B]2
The rate of the reaction,
2NO + Cl2 → 2NOCl is given by the rate equation, rate = k[NO]2[Cl2]
The value of the rate constant can be increased by
increasing the temperature
increasing the concentration of NO
increasing the concentration of the Cl2
increasing the concentration of the Cl2
Half-life period of a first order reaction is 1386 s. The specific rate constant of the reaction is
5.0 x 10-3s-1
0.5 x 10-2 s-1
0.5 x 10-3 s-1
0.5 x 10-3 s-1
For the reaction, A+B → Products, it is observed that
1) On doubling the initial concentration of A only, the rate of reaction is also doubled and
2) On doubling the initial concentrations of both A and B , there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is, given by
rate = k [A]2[B]
rate = k[A][B]2
rate = k[A]2[B]2
rate = k[A]2[B]2
