 Multiple Choice Questions
Multiple Choice QuestionsThe rate of a first-order reaction is 0.04 mol L-1s-1 at 10 sec and 0.03 mol-1L-1 s-1 at 20 sec after initiation of the reaction. the half-life period of the reaction is
34.1 s
44.1 s
54.1 s
54.1 s
The ionic radii of A+ and B- ions are 0.98 x 10-10 m and 1.81 x10-10 m. The coordination number of each ions in AB is
4
8
2
2
The activation energy of a reaction can be determined from the slope of which of the following graphs?
InK vs T
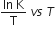


When initial concentration of a reactant is doubled in a reaction, its half-life period os not affected the order of the reaction is?
Zero
first
second
second
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 200C to 350C ?(R=8.314 J mol-1 K-1).
342 kJ mol-1
269 kJ mol-1
34.7 kJ mol-1
34.7 kJ mol-1
In a reaction, A+B → Product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentrations of both the reactants (A and B) are doubled. Rate law for the reaction can be written as
Rate = k[A][B]2
Rate = k[A]2[B]2
Rate = k[A][B]
Rate k[A]2[B]
In Freundlich adsorption isotherm, the value of 1/n is
between 0 and 1 in all cases
between 2 and 4 in all cases
1 in case of physical adsorption
1 in case of chemisorption
In a zero order reaction for every 10o rise of temperature, the rate is doubled. If the temperature is increased from 10oC to 100oC, the rate of the reaction will become
256 times
512
64 times
128 times
Activation energy (Ea) and rate constants (k1 and k2) of chemical reaction at two different temperatures (T1 and T2) are related by




Which one of the following statements for the order of a reaction is correct?
Order is not influenced by stoichiometric coefficient of the reactants
Order of reaction is sum of power to the concentration terms of reactants to express the rate of reaction
Order of reaction is always the whole number
Order of reaction is always the whole number
