 Multiple Choice Questions
Multiple Choice QuestionsWhen copper is heated with conc. HNO3 it produces
Cu(NO3)2 and NO
Cu(NO3)2, NO and NO2
Cu(NO3)2 and N2O
Cu(NO3)2 and N2O
Which one of the following statements is correct when SO2 is passed through acidified K2Cr2O7 solution?
The solution is decolourized
SO2 is reduced
Green Cr2(SO4)3 is formed
The solution turns blue
Because of lanthanoid contraction, of elements which of the following elements have nearly same atomic radii ? (Number in the parenthesis are atomic number).
Ti (22) and Zr(40)
Zr(40) and Nb (41)
Zr (40) and Hf (72)
Zr (40) and Hf (72)
In acidic medium,H2O2 changes Cr2O72- to CrO5 which has two (-O-O-) bonds. Oxidation state of Cr in CrO3 is
+5
+3
+6
+6
Reason of lanthanoid contraction is
negligible Screening effect of 'f' orbitals
increasing the nuclear charge
decreasing the nuclear charge
decreasing the nuclear charge
Which of the following does not give oxygen on heating?
KClO3
Zn(ClO3)2
K2Cr2O7
K2Cr2O7
Which of the following lanthanoid ions is diamagnetic?
(At. number; Ce =58, Sm =62, Eu =63, Yb = 70)
Ce2+
Sm2+
Eu2+
Eu2+
KMnO4 can be prepared from K2MnO4 as per reaction
The reaction can go to completion by removing OH- ions by adding
HCl
KOH
CO2
CO2
Which one of the following does not correctly represent the correct order of the property indicated aginst it?
Ti < V<Cr< Mn: increasing number of oxidation states
Ti< V<Cr3+<Mn3+ : increasing magnetic moment
Ti < V < Cr < Mn : Increasing melting points
Ti < V < Cr < Mn : Increasing melting points
Four successive members of the first series of the transition metals are listed below. For which one of them, the standard potential value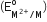 has a positive sign?
has a positive sign?
Co (Z=27)
Ni (Z=28)
Cu (Z=29)
Cu (Z=29)
