CBSE Multiple Choice Questions
Multiple Choice QuestionsA monoatomic gas at a pressure p, having a volume V expands isothermally to a volume 16 V. The final pressure of the is (take 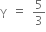 ),
),
64 p
32 p


Steam at 100o C is passed into 20 g of water at 10o C. When the water acquires a temperature of 80o C, the mass of water present will be,
24 g
31.5 g
42.5 g
42.5 g
Certain quantity of water cools from 70o C to 60o C in the first 5 min and to 54o C in the next 5 min. The temperature of the surroundings is,
45o C
20o C
42o C
42o C
A refrigerator works between 4o C and 30o C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is (Take 1 cal = 4.2 Joules)
23.65 W
236.5 W
2365 W
2365 W
A thermodynamic system undergoes cyclic process ABCDA as shown in the figure. The work done by the system in the cycle is: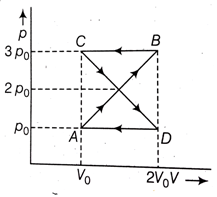
po Vo
2 po Vo
![]()
zero
The ratio of the specific heats 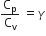 in terms of degrees of freedom (n) is given by
in terms of degrees of freedom (n) is given by
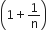
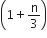
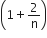
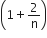
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced its half. Then,
compressing the gas through adiabatic process will require more work to b done
compressing the gas through isothermally or adiabatically will require the same amount of work.
which of the case (whether compression through isohermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
which of the case (whether compression through isohermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
A Carnot engine, having an efficiency of as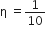 heat engine, is used as a refrigerator. If the work is done on the system is 10J, the amount of energy absorbed from the reservoir at lower temperature is
heat engine, is used as a refrigerator. If the work is done on the system is 10J, the amount of energy absorbed from the reservoir at lower temperature is
100J
99 J
90 J
90 J
Figure below shows two paths that may be taken by a gas to go from a state A to a state C. 
In process AB, 400 J of heat is added to the system and in process BC, 100 J of heat is added to the system. The heat absorbed by the system in the process AC will be
380 J
500 J
460 J
460 J
A piece of ice falls from a height h so that it melts completely. Only one-quarter of the heat produced is absorbed by the ice and all energy of ice gets converted into heat during its fall. The value of h is [Latent heat of ice is 3.4 x 105 J/kg and g =10 N/kg]
544 km
136 km
68 km
68 km

